Supercritical and Near Critical CO2 Micronization
CO2 Micronization and Atomization
Micronization systems are designed to produce small, very small particles and nanoparticles. The change in size depends on the technology used. Near-critical Expansion Atomization (NEA) produces 10 to 50 micrometer-sized particles, while Particles from Gas Saturated Solutions (PGSS) and Supercritical Anti-Solvent (SAS) can produce nanometer-sized particles. Supercritical Assisted Atomization (SAA) is a version of SAS that counteracts the decrease in temperature during expansion.
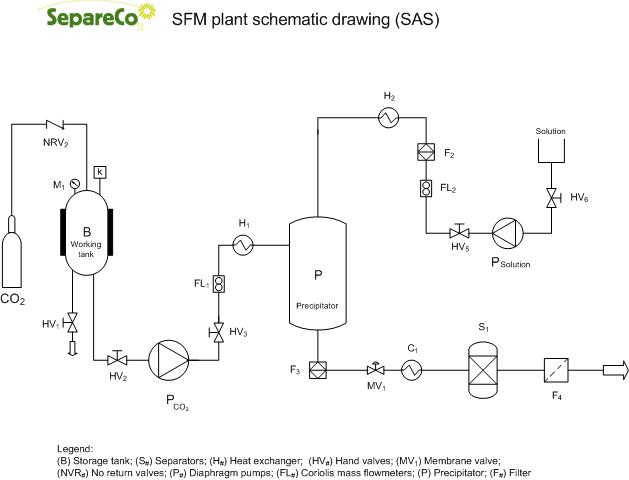
12 L Supercritical CO2 Micronizer SAS
20 L Near Critical CO2 Micronizer NEA
100 L Supercritical CO2 Micronizer PGSS
Supercritical CO2 Micronizer SAA
CO2 Micronization of oily ingredients
CO2 micronization is a process that reduces the size of particles of oily ingredients, such as fats, waxes, and lipids, to a very fine powder to enhance their physical and chemical properties. This technique can improve the solubility, stability, and bioavailability of these ingredients, as well as enhance their sensory properties and functionality.
Separeco method of micronization is using supercritical or near-critical carbon dioxide (CO2) as a solvent and antisolvent. This method is especially suitable for powders of oily ingredients, such as vitamins, antioxidants, and essential oils, because it avoids the use of organic solvents and high temperatures that may degrade the quality of the products. The process involves dissolving the oily ingredients in CO2 at high pressure and temperature, and then spraying the solution into a lower pressure chamber, where the CO2 rapidly expands and forms fine particles of the solute.
Micronization is establishing itself as one of the emerging technologies capable of modifying the biological response to the intake of active ingredients such as medicines in general, antibiotics, etc. well defined with the term bioavailability.
It is easy to find many solutions for the micronization of hydrophilic substances, but very few for lipophilic substances. In fact, until recently, it was possible to simply adsorb oils on maltodextrins or similar material.
Now, the formation of particles in the micrometer and nanometer scale is possible for the most part using the power of CO2.
We offer multiple solutions and technologies to produce micronized lipophilic and hydrophilic matrices. Among these are the Near-critical Expansion Atomization (NEA), the Particles From Gas-Saturated Solutions (PGSS) and the Supercritical Anti Solvent (SAS).
We can micronize polymers used for drug delivery, micronize antibiotics, coating with adjuvant substances that can protect the APIs from attacks by gastric juices and promote the absorption of medicinal substances. The formulation of the matrices to be micronized changes upon customer’s needs.



CO2 Micronization: Most known technologies
NEA (Ner-critical Expansion Atomization)
In NEA process, the carbon dioxide is used in this process for the atomization and the crystallization of the product. The product subjected to the process is maintained in the liquid phase in a feed tank at a controlled temperature and subsequently conveyed, at the desired pressure, to the “atomization tower” where there is contact with the carbon dioxide released to atmospheric pressure.
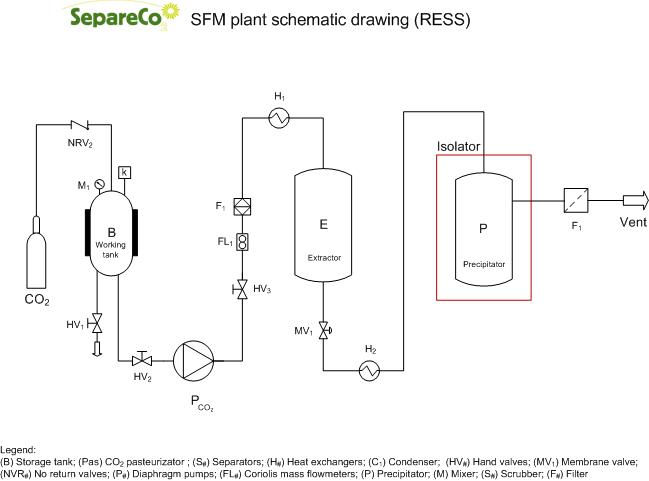
RESS (Rapid Expansion of Supercritical Solution)
RESS (Rapid Expansion of Supercritical Solution) is used generally to produce fine particles for the food, cosmetic and pharmaceutical industry.
Organic material is dissolved in supercritical carbon dioxide and is used to rapidly expand the supercritical solution through an expansion nozzle. Due to an abrupt decrease of pressure to atmospheric, very high Supersaturation values is achieved which led to small particle sizes.
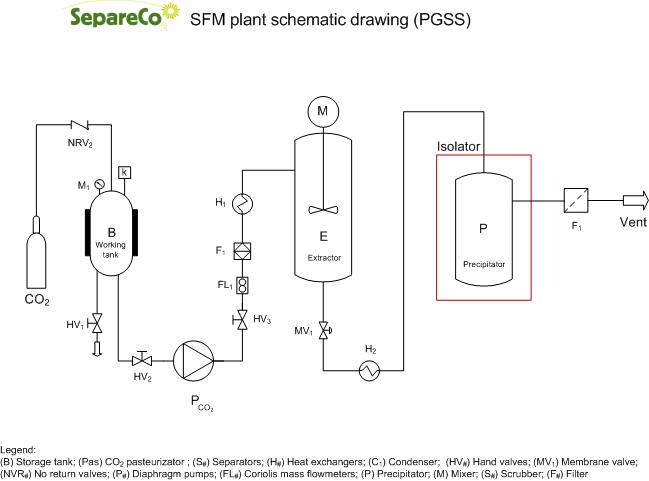
PGSS (Particles from Gas Saturated Solutions)
PGSS is a technique for the production of microparticles of different materials of relatively low melting temperatures, such as polymers, waxes or fats. The process is based on the capacity of those materials to dissolve large amounts of CO2 at moderate pressures. Upon depressurization down to ambient conditions, the dissolved CO2 is rapidly released and expanded, producing an intense cooling effect that promotes the formation of microparticles.
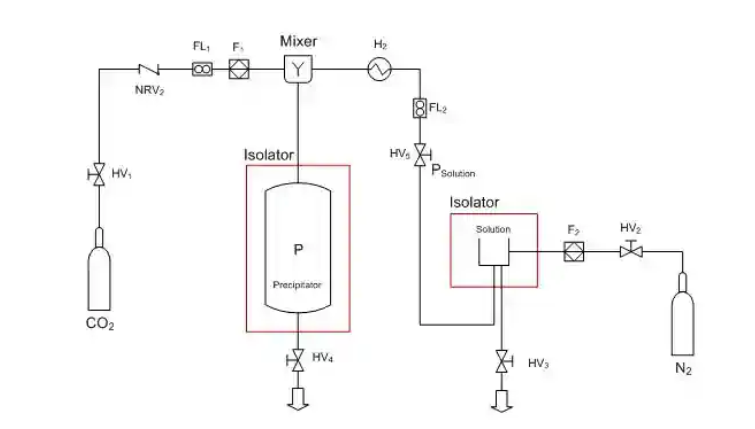
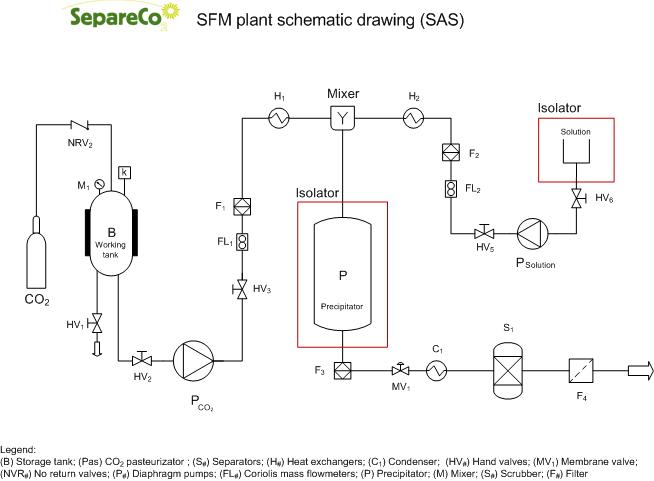
SAS (Supercritical Anti Solvent)
SAS, according to its name, applies the supercritical fluid as an antisolvent. Hence the solute to be micronized has to be quasi non-soluble in the supercritical fluid. This process is structured in a differently way than previous RESS and PGSS. The SCF is first pumped to the top of the high pressure vessel until the system reaches a constant temperature and pressure. Subsequently, active substance solution is sprayed as fine droplets into above SCF bulk phase through an atomization nozzle.
SAA (Supercritical Assisted Atomization)
SAA process is focused on the nebulization of the liquid solution rather than using dense gas (SCF) to achieve precipitation by solubility reduction for the solute to be micro- or nano-sized. At first, the solute is dissolved or suspended in aqueous or organic solvent or their mixture and then mixed intimately with near critical or SC by pumping both fluid through a near zero volume tee to generate an emulsion. The resultant emulsion is rapidly expanded through a flow restrictor to near atmospheric pressure to form aerosol consisting of micro droplets and micro bubbles.