







25+ years of experience in supercritical CO2 equipment.
WE MEET CUSTOMERS NEEDS.
Turn key systems, on site and remote assistance, pre-sale and after-sale support, financing consultancy, process setup, matrix tests, commissioning, startup and maintenance programs.
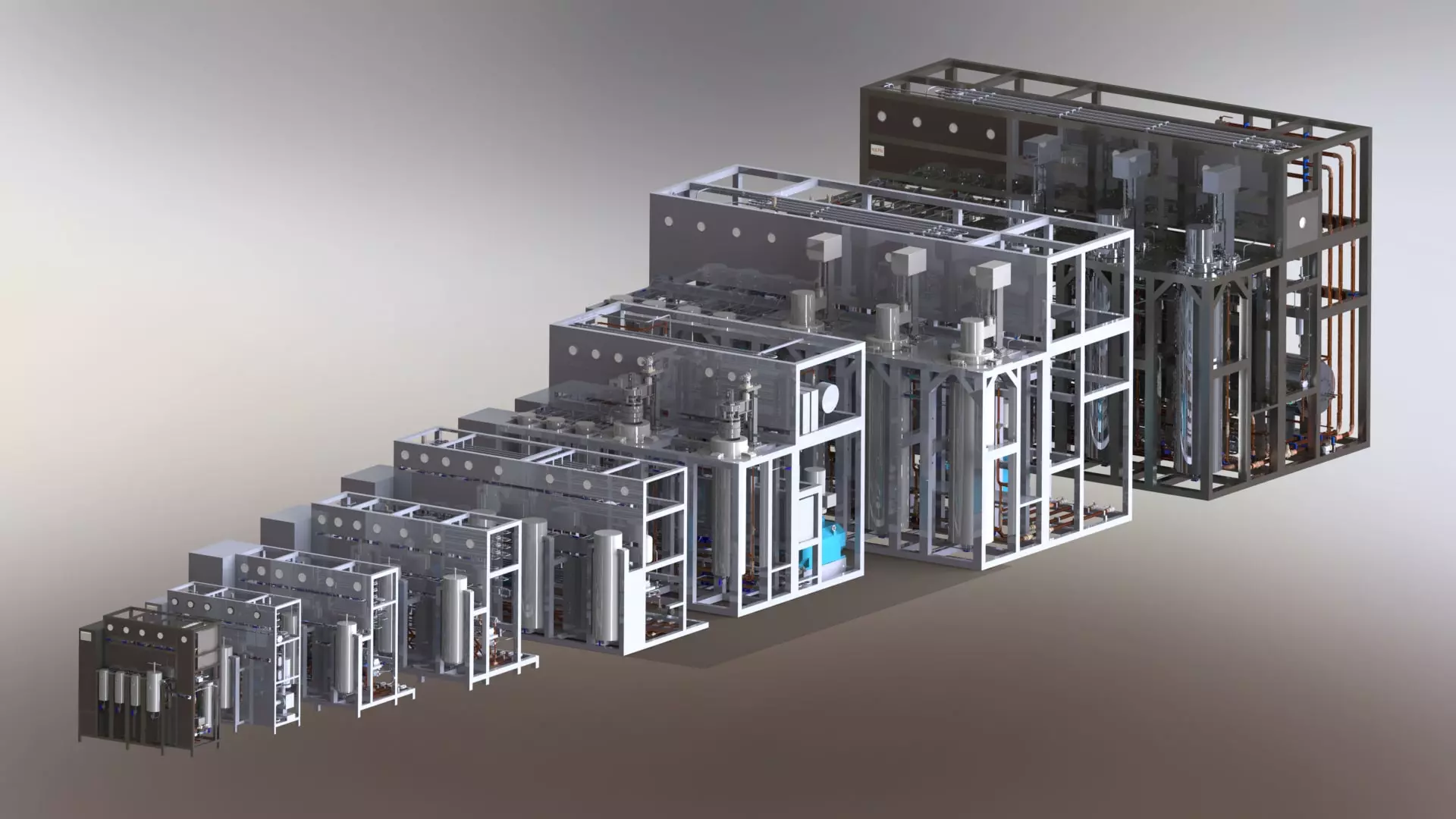
Supercritical CO2 equipment families
Supercritical CO2 extraction equipment
SUPERCRITICAL CO2 EQUIPMENT
Separeco has the widest range of extraction, separation, micronization and chromatography systems available on the market. We have in our catalog more than 26 different models in production and over 40 special ones built on URS (User Requirement Specifications).
They are all equipped with process automation that works with recipes (real software programs) that are loaded depending on the raw material to be processed. Recipes are available for over 300 raw materials both for extraction (processes with one, two or three extractors – standard, semi-continuous, overlapping) and for fractionation, micronization and chromatography.
SOLUTIONS AND APPLICATIONS
Separeco offers solutions for many industries and research centers. Our solutions can be applied to different technological sectors, both in production and in industrial research.
In most cases these are turn key solutions, in other cases they are very specific solutions applied in particular cases, such as the micronization or atomization of powders or the removal of flammable solvents or the isolation of ingredients.
TECHNICAL SERVICES
If you have not enough experience in the extraction with supercritical fluids, it is difficult to fine-tune the extraction process of your matrix . At Separeco we are organized to test new matrices for our customers and analyze the extract.
Separeco helps customers to correctly prepare the matrix by indicating the degree of humidity, grain size and pre-treatment and suggests process conditions such as the pressure and temperature and, very importantly, the residence time, controlled by the CO2 flow rate.

FINANCING
Our supercritical CO2 equipment financing options are designed to help businesses across all industries efficiently invest in new assets.
Our indstrial finacing partner promotes the sale of products of the Italian companies on the international markets, thus contributing to expand indirectly the target markets of the Italian manufacturers all over the world.
Contact us for more information.


EXPLOSION PROOF
Separeco manufactures and supplies explosion-proof equipment and accessories for industrial environments at risk of explosion.
Electrical panels, junction boxes, cable glands, control stations, pipe unions, plugs and sockets, lighting fixtures are manufactured according to international standards such as UL, ATEX, NFPA70, IECEX to meet safety requirements in the industrial sector.
Certification for ATEX Zone 1 and 2 for countries accepting European regulation or Class 1 – Division 1 and 2 for North America. Be careful before purchasing.
North America countries use NPFA70/NEC 500 directive that is not accepted in European Economic Area (EEA).
Conversely, in European Economic Area (EEA) ATEX directive is adopted and NPFA70/NEC 500 directive is not accepted.






CERTIFICATIONS
We offer:
more CERTIFICATIONS and STANDARDS to guarantee more SAFETY and QUALITY rigorous DESIGN, standard or based on URS* careful CONSTRUCTION, VERIFIABLE by the customer.
Our certifications:
- TUV ISO9001:2015
- CE Europe
- Apave PED
- UL/CSA listed
- ASME U stamp
- CRN Canada
- Ex/IECEx Explosion proof
* URS (User Requirement Specifications).



GMP
A Good Manufacturing Practice (GMP) is a production and testing practice that helps to ensure a quality product. GMP guideline give us a guidance for the manu-facturing of active Pharmaceutical Ingredients (APIs) under an appropriate system for managing quality.
A Good Automation Manufacturing Practice (GAMP) focuses on the whole system and the end product, where as the FDA focuses on each process and stage of pro-duction that contributes to the end product. FDA guidance are incorporated into the GAMP guidelines.
Specifically, title 21 CFR Part 11 is the part of title 21 of the Code of Federal Regulations that establishes the United States Food and Drug Administration (FDA).
