Processo statico-dinamico (PSD).
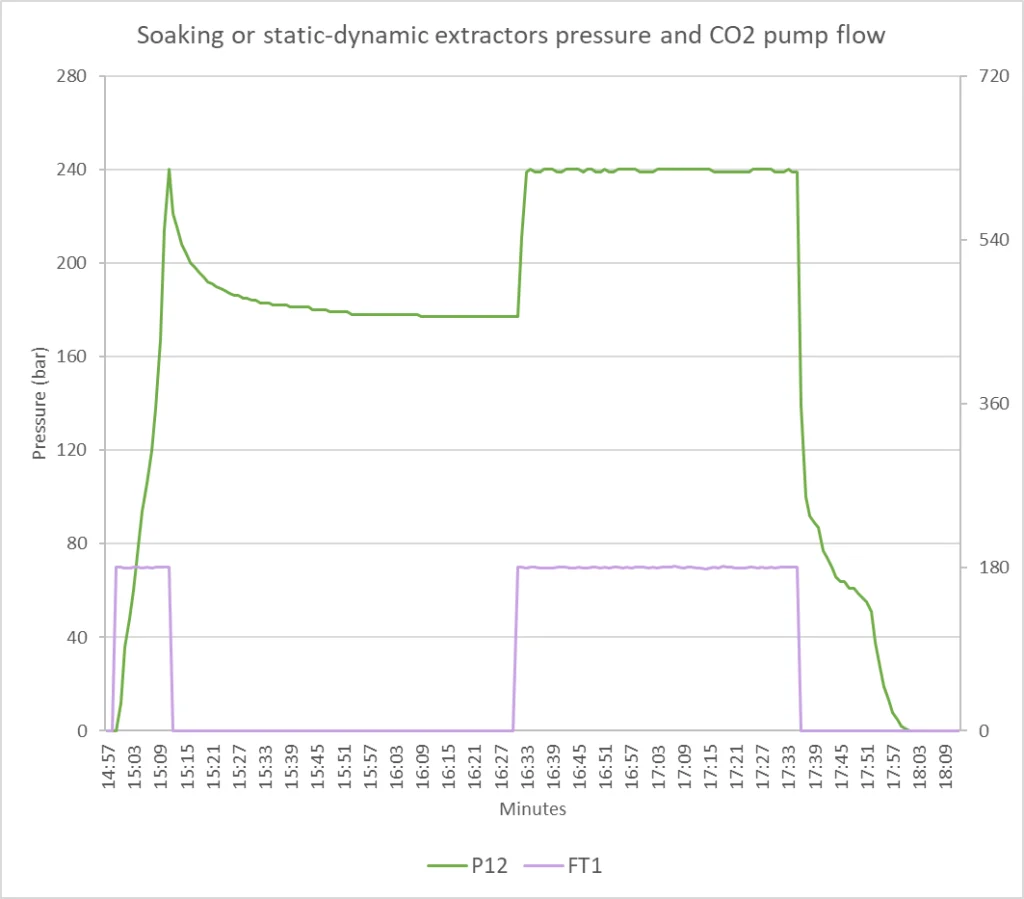
The soaking extraction or Static-Dynamic Process (SDP) allows the supercritical CO2 to remain in contact with the raw material for the time necessary to dissolve the target compound. The raw material is brought to the set point pressure and, when reached, the system stops the pump and isolates the extractor. The system then passes to another extractor leaving the matter in the first extractor to absorb the CO2. Soaking extraction resolves problems of slow extraction kinetics and high mass transfer resistance with great effectiveness, increasing efficiency by soaking material before extraction CO2 restarts again.
The soaking cycle works in this way: when the extraction phase for extractor 1 is completed, the CO2 is transferred from extractor 1 to extractor 3. The pressure in extractor 1 is decreasing very fast and the pressure in extractor 3 is increasing very fast. Immediately after the cross point (blue-red), the pump starts to run therefore the pressure in extractor 3 increases and the extractor 1 is under venting phase. When the pressure in the extractor 3 is reached the pump stops and the soaking process takes places. When the pump starts to run again the extraction process the pressure in extractor 2 increases and the extractor process begins.
Soaking / static-dynamic extraction in the scientific literature.
The transport mechanism that occurs in supercritical fluid extraction is considered a leaching process. In leaching, the solvent must first travel to the surface of the material and diffuse through the pores. The solute then dissolves in the solvent, and is transported to the surface of the particle. Finally, the solute is transferred into the bulk fluid. This process will proceed until an equilibrium concentration of the solute is reached in the bulk fluid.
The use of static-dynamic cycling in supercritical fluid extraction is similar to an equilibrium-staged separation. In essence, each static-dynamic cycle simulates a stage of the separation. During the static soak time, the system is allowed ample time to reach equilibrium, and then released during the dynamic phase. By performing this extraction process in cycles, equilibrium stages are reached allowing for an efficient extraction that uses half the amount of CO2 that would be used in a continuous system.
Ref: Megan Matricardi, Robert Hesketh, Stephanie Farrel, Rowan University, NJ
