Diagramma di fase della CO2 - Stati della materia.
CO2 isn’t a liquid at room pressure: it’s a gas.
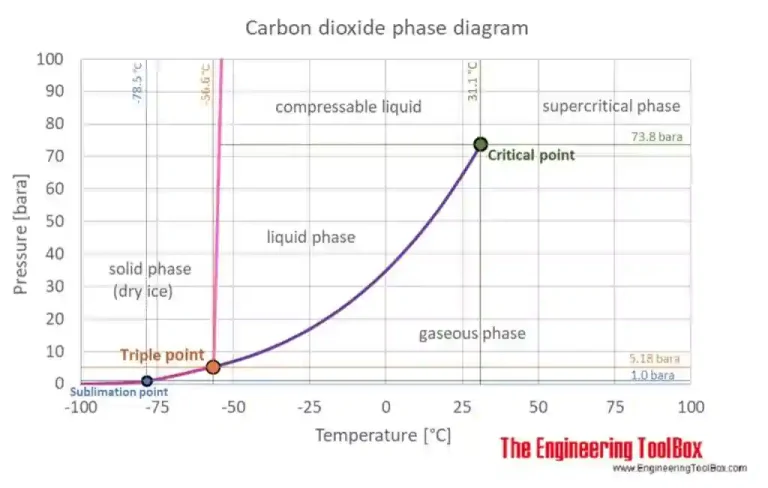
In the picture here on the right, taken from The Engineering ToolBox, the equilibrium curve is the colored one between the triple point and the critical point.
The Triple Point is the only plot’s point in which there are at the same time solid, liquid and gasseous phase of the material. It’s characterized by particular condition of temperature and pression. The Critical Point is characterized by the disappearance of the difference between gaseous phase and liquid phase.
In condiction of temperature and pressure over the critical point, we talk about Supercritical Fluid. The CO2 is at the same time a liquid a gas during all of the plot’s points that shape the Equilibrium Curve.
Going over other thermodynamic and technical discussions, maintaining the CO2 always along this curve will save a lot of energy. Our system is designed to maintain this equilibrium in the Reservoir, one of the most important vessel in the system.
Without a good control of temperature and pressure at the reservoir’s level, you’ll never be able to have a stable flow in the system.
In our system it’s easy to set the pressure in the reservoir at the desired value. We suggest 48 bar. The system, thanks to the full automation control, will stabilize the pressure automatically.
