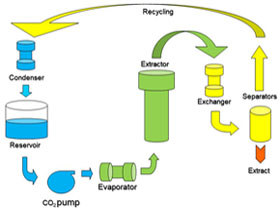
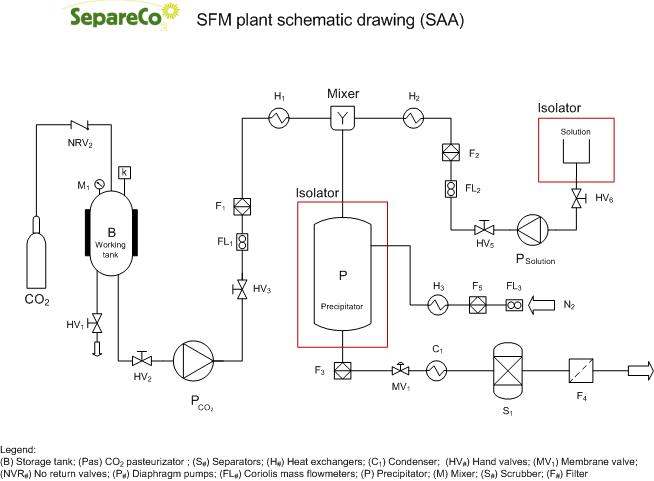
A liquid co-solvent can be added to CO2 to increase its solvent power on polar molecules.
Indeed, supercritical CO2 is a good solvent for lipophilic non-polar compounds like oils, whereas, it has a low affinity with hydrophilic polar compounds like sugars.
Process engineers often add small quantities of liquid solvents (for example, ethyl alcohol) that are readily solubilized by supercritical CO2 to extract poliphenols or other compound with intermediate polarity.
When in solution, they modify the solvent power of supercritical CO2. This strategy has the drawback that, a larger solvent power could also mean lower process selectivity and since the co-solvent is liquid at atmospheric pressure, it will be collected in the separators together with the extracted compounds.
All oils are very non-polar compounds. CO2 is an non-polar solvent, so there is a good affinity between them. Solvents are classified according to a scale of polarity depending on their ability to dissolve polar or non-polar molecules. Water is the most polar solvent, dissolving all kinds of compounds that can be ionized or that contains hydrophilic moieties like sugars, proteins, amino-acids,….
Organic solvents, like light alkanes (hexane, heptane,…) or chlorinated hydrocarbons, do not dissolve these compounds, but only hydrophobic molecules that are not at all soluble in water like fats, oils, hydrocarbons, essential oils,… : therefore they are called non-polar solvents.
Other solvents like alcohols, amines, ketones,…exhibit intermediate behavior. Most supercritical fluids behave like non-polar solvents exhibiting a strong affinity with lipids and hydrocarbons, but a weak affinity with oxygenated or hydroxylated molecules.
By adding a polar or medium polar co-solvent (ethanol or light alcohols, esters or ketones) in the right percentage and for a correct and pre-determinated time during extraction, it is possible to fine tune total polarity of the supercritical solvent.
Entraining agents have different properties than supercritical CO2. The critical point (CP) of CO2 is 73.8 bar and 31.5° C; ethanol’s CP is 63 bar and 241° C. A mathematical description requires computational chemistry to show how the entraining agents interact with the CO2. Suffice it to say, CO2 will be in the supercritical phase and the entraining agents will be in the liquid phase. This changes the solvent characteristics of the CO2 and improves extraction yields.