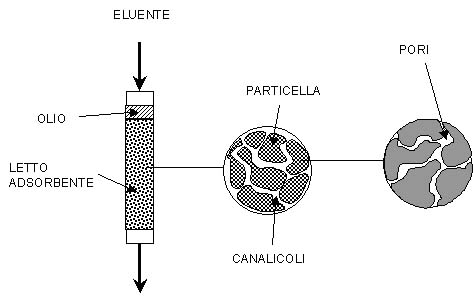
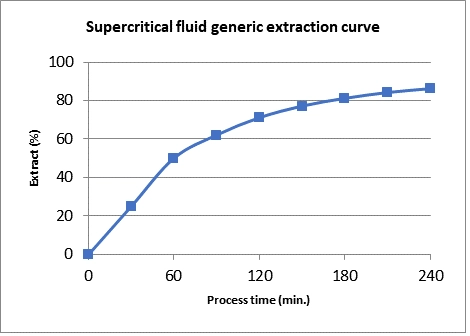
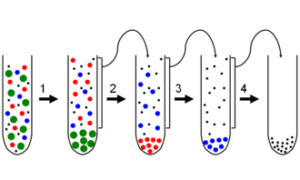
Processus de séparation et de fractionnement.
After the extraction process, the mixture composed by CO2 and solutes leaves the extraction vessel(s) and it is directed to the separation vessels. By varying the pressure, flow and temperature of these vessels, it is possible to induce the selective precipitation of different chemical compounds as a function of their different saturation conditions in the supercritical fluid.
First of all, the mixture is directed in first separator and here, with the use of a particular valve, which is called Lamination Valve, the pressure goes down and (for the effect of Joule-Thomson Effect the rapidly-expanded gas during depressurization process cools because the molecules get the energy using their specific heats), also the temperature of gaseous CO2 drops dramatically, and two important effects are observed:
- Solvent properties of CO2 change istantly: Density of the fluid is reduced 10 fold and the expansion of the CO2 changes the speed of the CO2 from centimeters per second to meters per second.
- All compounds dissolved in the CO2 immediately fall out of solution because the fluid changed its status from supercritical to gaseous and it has become a weak solvent.
First separator is a heated gravimetric separator. This design is particular effective for the heaviest compounds of the extract. The gravimetric separator works by gravitational force. But this operation isn’t enough to completely clean up the CO2, in fact other lighter solutes are not separated from the solvent in this separator. For this reason there are other 2 separator in series.
Second separator is a heated cyclonic separator. While the first works by gravity force, the second works by centrifugal force. The fluid moves very quickly creating a vortex inside the vessel. The fluid continues to spin and the particles of extract begin to separate moving toward the wall of the separator, sliding to bottom. The temperature of the wall determines which compound will be condensed. Generally, essential oils, like hydrocarbon terpenes, can be found in this separator, as they are lipid-soluble compounds, while oxygenated terpenoids and sterols travel to the third separator dissolved in water micro drops as they are polar compounds and the condensation point (because of heating) is too high to condense them in the second separator.
The third is a cooled cyclonic separator. Micro droplets of water will condense and collect in this separator along with hydrophilic/oxygenated terpenes and other volatile substances due to their low condensation point.