Supercritical CO2 Chromatography Equipment
Supercritical CO2 chromatography (SFC) is a technique that uses carbon dioxide in its supercritical state as the mobile phase to separate and analyze different compounds. Supercritical CO2 has the advantages of low viscosity, high diffusivity, and tunable polarity, which make it suitable for fast and efficient separations. Supercritical CO2 chromatography can be applied to various fields, such as pharmaceuticals, natural products, environmental analysis, and food science.
500 mm column Supercritical CO2 Chromatography
800 mm column Supercritical CO2 Chromatography
Supercritical Fluid Chromatography (SFC)
Supercritical Fluid Chromatography (SFC) is a fractionation technique widely utilized in the pharmaceutic industry, where productions are highly valuable, because it is a very efficient technique, and it allows to separate compounds which would be not isolated with techniques such as distillation or solvent extraction. There are different kinds of chromatography, but substantially they all utilize the ability of a Porous Solid (stationary phase) to selectively bind the compounds to be isolated, the latter carried by a Fluid (mobile phase). Such phenomenon is called Adsorption. Supercritical elution chromatography is particularly utilized for the fractionation of esters mixtures having similar molecular weight.
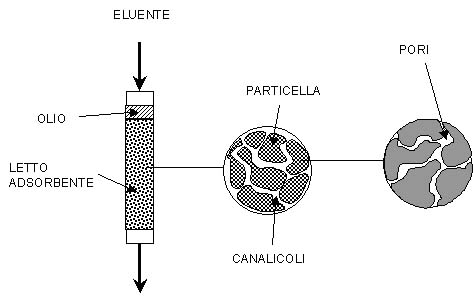
The substantial difference between the traditional chromatography and the supercritical one is that, while in the first type the eluent is a gas or a liquid solvent, in the second type the mobile phase is a supercritical fluid. This figure on the right shows with increasing detail level, a chromatographic system. The mixture to separate is injected in the column and it get absorbed in the first trait of the stationary phase.
Then the eluent is made flow, and in the supercritical fluid chromatography (SFC) this contributes to the substances separation. In the case of the system SC CO2-oil, for example, at the operational temperature and pressure, the solvents is dissolved in the oil bulk, blowing it up and originating a Biphasic System, in which a CO2 rich phase is in the supercritical state, whereas the other, rich in oil, is liquid. The eluent flow perturbs the equilibrium state and generates concentration gradients between the liquid phase, the supercritical phase and the solid phase, so there are material exchanges tending to bring the system to the equilibrium.
In particular there is a flow between the supercritical and the liquid phases that, in strict contact with each other, move countercurrent along the channels.