Come funziona l'estrazione con la CO2?
The main feature of supercritical fluids (SCF) is the tunability of their properties as solvent, supercritical CO2 or SC-CO2 extraction processes present an important advantage over low pressure methods, i.e., the selectivity of supercritical CO2 can be adjusted by varying temperature and pressure to obtain fractions containing specific compounds. Since the enhanced solubility effect is selective, a supercritical fluid can form the basis of a system for separating components of mixtures. At the same time, vapor pressure effects are also enhanced.
The recovery of extracts concentrated in target compounds, even during the extraction process, can lead to a product with high economic value and simplify subsequent fractionation steps.
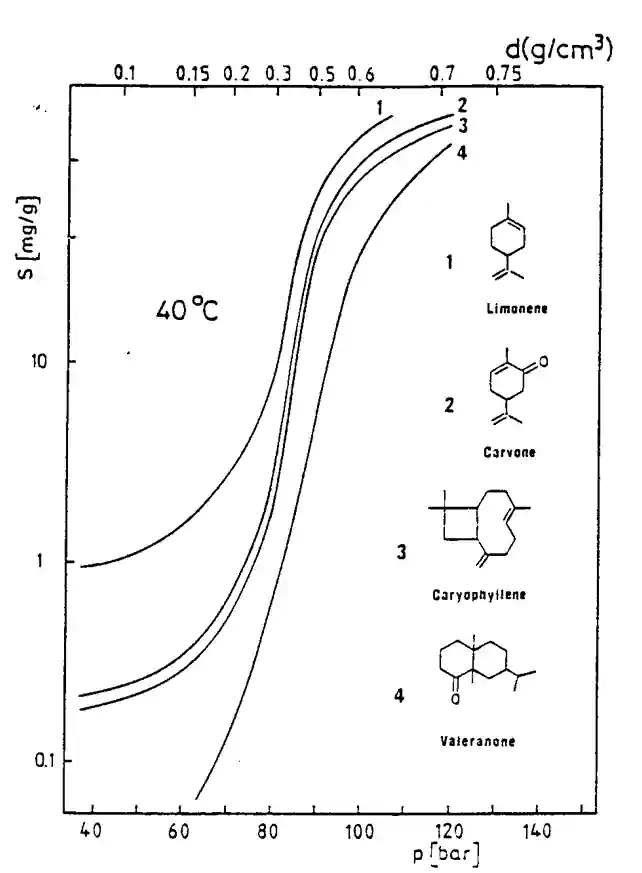
As the supercritical CO2 density increases, its transport capability also increases, to the point that it can entrain significant amounts of relatively nonvolatile compounds. Under proper conditions, supercritical CO2 separations combine the phenomena of solvent extraction and distillation. The behavior of supercritical substance changes with a rise of its density. In particular, it can dissolve substantial quantities of compounds that would be at best sparingly soluble in the same substance in its ordinary liquid or gaseous states.
The supercritical method has the advantage that it can be used for separating mixtures of high-boiling components that would decompose during fractional distillation.
Another strong point of supercritical CO2 extraction is that once the oil is extracted from the plant material, the CO2 is simply returned to its gaseous state by lowering its pressure, allowing the gas to quickly and completely dissipate.
The supercritical CO2 extraction and purification process is also more important because it supports the separation without any contamination or degradation.
Recently many researchers have proved that the performance of the supercritical fluid technology increases with the use of alternative solvents or addition of the modifiers to SC-CO2 because it has limitations resulting from its lack of polarity and associated lack of capacity for specific solvent–solute interactions. Thus, there is a great incentive to improve solvent polarity. For these purposes, small amounts of a highly polar co-solvent can be added to CO2 in order to increase its solvating power.
