Frazionamento in fluidi supercritici (FFS).
For Fractionation it is meant the separation of one or more components (from a mixture) with the employment of a miscible or immiscible solvent, thanks to a favorable repartition coefficient of the solute. The repartition coefficient is given by the ratio between the solute concentration in the SCF at the equilibrium and the concentration of the solute in the starting matrix at the equilibrium.The extraction is a convenient process only if the component to extract shows simultaneously:
- A favorable repartition coefficient (meaning a high solubility in the SCF);
- A favorable separation factor (if evaluated in relation to possible compounds being extracted from the mixture together with the compound of interest).
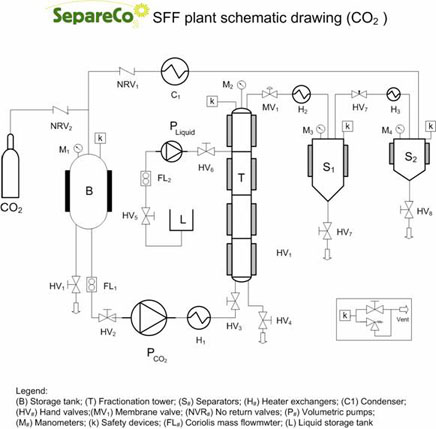
In the other cases, in order to obtain a satisfactory separation level, it is necessary to recur to the fractionation process, modifying the Fractionation Tower (or Column) configuration (see figure): the column is divided in a Rectification Section (the portion of the column above the feeding section) and an Exhaustion Section (the portion under the feeding section). The fractionation can occur in two different ways: by temperature gradient and by extract reflux. The choice of one of the two methods is generally conditioned by the plant design.
If the tower is equipped with a heating system differentiated along the column height, it is convenient to realize the temperature gradient fractionation with the tower heated at different temperatures, generally increasing along the height. The different compounds’ solubility in SC-CO2 decreases, most of the times, with the temperature increase. In the exhaustion section, where the temperature is lower, there will be a rough but efficient solubilization of the compounds to fractionate. In the rectification section instead, temperature is set properly in order to provoke a drastic decrease in the solubility of one of the two compounds. This will be released by the SC-CO2 and it will undergo an internal reflux, while the most soluble compound will be concentrating in the extract. This kind of fractionation allows to modulate the selectivity factor (within certain limits) modifying the temperature in relation to the exhaustion section.
Anyway it is necessary to know in advance the solubility of the compounds to separate in SC-CO2 in relation to the temperature. The reflux fractionation of the extracts occurs at constant temperature, but it needs the insertion of a second pump for the liquids before the separator, in order to recirculate part of the extracts toward the column head. In this way, therefore, even if the separation factor is not very favorable, it is possible to obtain the fractionation, since the mixture fed is more and more rich in the desired component. Regulating the extracts fraction to recycle and the number of recycles to apply, it is possible to obtain a total extract having the desired composition.In the case in which the separation factor is such to forbid separation with extraction mode or, instead, extract richer than the possible limit reachable are requested, it is necessary to apply reflux fractionation. In the case of SFF the extracting phase is constituted by SC-CO2, which might be added of a co-solvent.
Fractionation principles of ethylesters in tower.
- Let’s suppose, for simplicity reasons, that the ethylesters mixtures are composed by two pseudocomponents, meaning groups of molecules with similar behavior:
- Light ethylesters, being 14-18 C fatty acids;
- Heavy ethylesters, being 20-22 C fatty acids.
Since the first group of compounds is more soluble in SC-CO2, it derives that an increase in the concentration of a heavy ethylesters mixture, it means to remove in the most efficient way the light ethylesters. Such removal can be conveniently performed utilizing Filling Towers, in which a solvent phase and a liquid phase (ethylesters mixture) come in touch countercurrent. The filling tower is the equipment usually utilized for liquid mixtures fractionation with liquid or gaseous solvents. The name tower (or column) comes from the fact that it appears to be like a recipient having a high height-diameter ratio. In general the fractionation is performed feeding continuously and countercurrent the mixture to separate and the extraction solvent. In order to maximize the contact between the phases and the mass exchange, inside the tower there is the filling, made of elements having proper size and/or shape (non structured filling ).
When the filling tower is at regime, the liquid mixture to fractionate, fed at the top of the tower, Moves Down along the filling delivering the most soluble compounds to the extraction solvent, which is lighter and moves along the tower in the opposite direction. The exhausted fraction (refined) is collected from the bottom of the tower, and the extract (composed by the solvent and the solubilized products) is collected from the top. In other words, in the case of ethylesters mixtures the lighter fraction of the mixture will be collected as extract at the top of the tower (being more soluble in the supercritical phase), while the heavier fraction (less soluble) will be collected from the bottom as refined, since it will concentrate in the residual liquid phase as an effect of the light fraction removal. Therefore it is to be considered that the more the similarity between the compounds to separate, the lower will be the productive capacity of the plant (expressed as Kg fractionate/column section), and the higher will be the height of the tower needed for the requested separation.
