¿Qué es un fluido supercrítico?
A supercritical fluid is any substance at temperature and pressure above its critical point. It can diffuse through solids like a gas, and it can dissolve materials like a liquid. In addition, close to the critical point, small changes in pressure or temperature result in
Large Changes in Density, allowing many properties of a supercritical fluid to be “fine-tuned”. Supercritical fluids are suitable as a substitute for organic solvents in a range of industrial and laboratory processes. Carbon dioxide and water are the most commonly used supercritical fluids, being used for decaffeination and power generation, respectively.
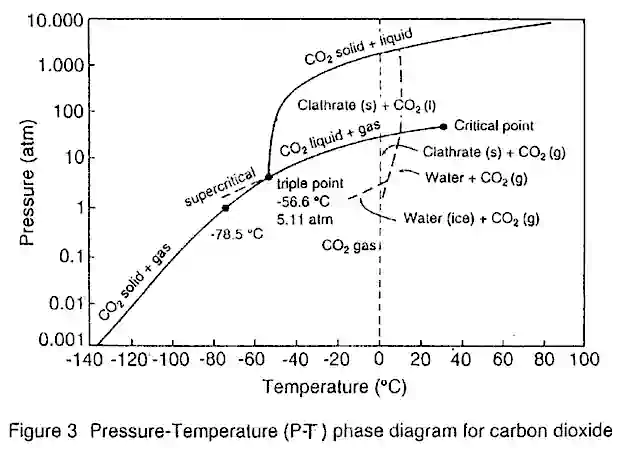
Supercritical Fluid Extraction (SFE) is the process of separating one component (the extractant) from another (the matrix) using supercritical fluids as the extracting solvent. If you need additional information, click here. Extraction is usually from a solid matrix, but can also be from liquids. SFE can be used as a sample preparation step for analytical purposes, or on a larger scale to either strip unwanted material from a product (e.g. decaffeination) or collect a desired product (e.g. essential oils). Carbon dioxide (CO2) is the Most Used supercritical fluid, sometimes modified by co-solvents such as ethanol or methanol. Extraction conditions for supercritical CO2 are above the critical temperature of 31°C and critical pressure of 74 bar. Addition of modifiers may slightly alter this. The discussion below will mainly refer to extraction with CO2, except where specified.
The Critical Point (C) is marked at the end of the gas-liquid equilibrium curve, and the shaded area indicates the supercritical fluid region. It can be shown that by using a combination of isobaric changes in temperature with isothermal changes in pressure, it is possible to convert a pure component from a liquid to a gas (and vice versa) via the supercritical region without incurring a phase transition.
The behavior of a fluid in the supercritical state can be described as that of a very mobile liquid. The solubility behavior approaches that of the liquid phase while penetration into a solid matrix is facilitated by the gas-like transport properties. As a consequence, the rates of extraction and phase separation can be significantly faster than for conventional extraction processes. Furthermore, the extraction conditions can be controlled to effect a selected separation. Supercritical fluid extraction is known to be dependent on the density of the fluid that in turn can be manipulated through control of the system pressure and temperature. The dissolving power of a SCF increases with isothermal increase in density or an Isopycnic (constant density) increase in temperature. In practical terms this means that a SCF can be used to extract a solute from a feed matrix as in conventional liquid extraction. However, unlike conventional extraction, once the conditions are returned to ambient the quantity of residual solvent in the extracted material is negligible.
The basic principle of SCF extraction is that the solubility of a given compound (solute) in a solvent varies with both temperature and pressure. At ambient conditions (25°C and 1 bar) the solubility of a solute in a gas is usually related directly to the vapor pressure of the solute and is generally negligible. In a SCF, however, solute solubilities of up to 10 orders of magnitude greater than those predicted by ideal gas law behavior have been reported.
The dissolution of solutes in supercritical fluids results from a combination of vapor pressure and solute-solvent interaction effects. The impact of this is that the solubility of a solid solute in a supercritical fluid is not a simple function of pressure.
Although the solubility of volatile solids in SCF is higher than in an ideal gas, it is often desirable to increase the solubility further in order to reduce the solvent requirement for processing. The solubility of components in SCFs can be enhanced by the addition of a substance referred to as an entrainer, or co-solvent. The Volatility of this additional component is usually intermediate to that of the SCF and the solute. The addition of a co-solvent provides a further dimension to the range of solvent properties in a given system by influencing the chemical nature of the fluid.
Co-solvents also provide a mechanism by which the extraction selectivity can be manipulated. The commercial potential of a particular application of SCF technology can be significantly improved through the use of co-solvents. A factor that must be taken into consideration when using co-solvents, however, is that even the presence of small amounts of an additional component to a primary SCF can change the critical properties of the resulting mixture considerably.
